GDAP1
The GDAP1 gene encodes ganglioside-induced differentiation-associated protein 1, a mitochondrial outer membrane protein essential for maintaining mitochondrial dynamics and peripheral nerve health.
Mutations in GDAP1 are a recognized cause of Charcot-Marie-Tooth disease, contributing to both demyelinating and axonal neuropathies characterized by progressive weakness, sensory loss, and motor dysfunction.
What is GDAP1 (Ganglioside-Induced Differentiation-Associated Protein 1)?
The GDAP1 gene encodes the ganglioside-induced differentiation-associated protein 1 (GDAP1), a mitochondrial outer membrane protein essential for maintaining the health and function of peripheral nerves.
GDAP1 is implicated in the regulation of mitochondrial dynamics and calcium homeostasis.
GDAP1 dysfunction is associated with several inherited neuropathies, most notably Charcot-Marie-Tooth disease (CMT), a common cause of progressive peripheral neuropathy.
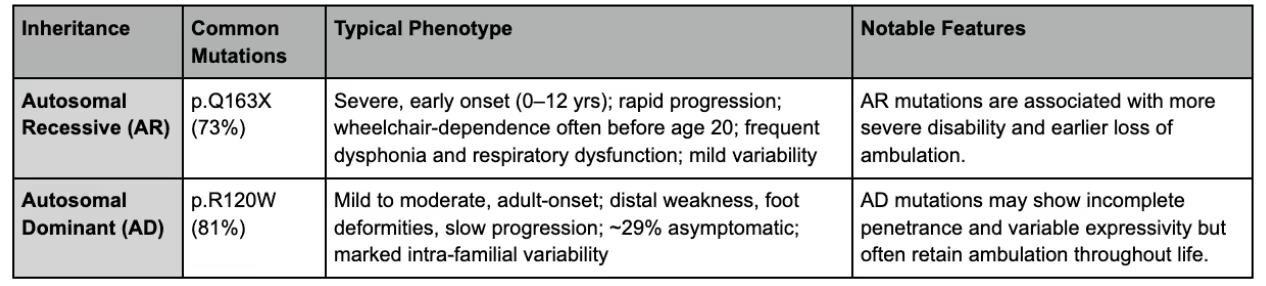
Two main clinical subtypes linked to GDAP1 mutations are CMT Type 4A (CMT4A), an autosomal recessive demyelinating neuropathy, and CMT Type 2K (CMT2K), an autosomal dominant axonal neuropathy.
GDAP1: A Protein Involved in Mitochondrial Dynamics
The GDAP1 gene is located on chromosome 8q21.11. It encodes a protein structurally related to the glutathione S-transferase (GST) family, but GDAP1 lacks confirmed enzymatic GST activity. Instead, GDAP1 has adopted a structural role, functioning as a mitochondrial outer membrane protein.
The protein contains two GST-like domains and a C-terminal transmembrane segment that anchors it to mitochondria.
GDAP1 is highly expressed in neuronal tissues, where it plays a critical role in supporting mitochondrial health and neuronal function.
Mitochondrial Function
GDAP1 is involved in mitochondrial dynamics, which are essential for maintaining mitochondrial networks and proper energy distribution within cells. This is especially critical in neurons due to their long axons and high metabolic demands.
GDAP1 is also involved in mitochondrial trafficking, helping mitochondria move to where they are needed within the cell. Recent evidence suggests that GDAP1 may interact with the cytoskeleton and participate in redox-sensitive processes.
Role in Neurons
Proper function of GDAP1 is essential for maintaining the structure and function of peripheral nerves.
GDAP1 mutations disrupt mitochondrial cristae organization, increase oxidative stress, and impair neural cell viability, highlighting the critical role of GDAP1 in motor neuron survival.
When is GDAP1 Genetic Testing Relevant?
GDAP1 genetic testing is most relevant when evaluating individuals with suspected CMT, particularly those with signs of CMT4A or CMT2K.
Typical features include progressive distal muscle weakness and wasting, often beginning in the feet and hands, sensory loss, and foot deformities such as pes cavus (high arches).
In autosomal recessive forms, motor milestones may be delayed in childhood.
Testing should also be considered in individuals with a family history suggestive of CMT. Identifying GDAP1 mutations can help confirm a diagnosis, guide prognosis, and inform family members about carrier status and reproductive risks.
In addition, GDAP1 testing is valuable when differentiating between other inherited neuropathies and various CMT subtypes, especially when distinguishing axonal from demyelinating patterns on nerve conduction studies.
Genetic Counseling
Genetic counseling is recommended both before and after GDAP1 testing. Counselors can help patients and families understand inheritance patterns, implications of test results, and options for reproductive planning.
What Do Specific GDAP1 Mutations Mean?
Different mutations in the GDAP1 gene may carry varying clinical significance:
Clinical Significance of Mutations
Identification of pathogenic GDAP1 mutations confirms a genetic diagnosis of CMT4A, CMT2K, or an intermediate form of CMT.
CMT4A typically follows an autosomal recessive inheritance pattern, requiring mutations in both copies of the GDAP1 gene, while CMT2K is usually autosomal dominant and may present even if only one gene copy is affected.
Genotype-Phenotype Correlations
Generally, autosomal recessive mutations (CMT4A) cause more severe disease with an earlier onset, often beginning in childhood and progressing rapidly, sometimes leading to wheelchair dependency and respiratory complications.
CMT2K mutations are usually inherited in an autosomal dominant fashion; this tends to have a milder course, often with adult-onset symptoms and variable severity within families.
Test Procedure and Interpretation
Testing for GDAP1 is performed as a genetic test to look for mutations in the gene that would alter functional protein availability. The following section outlines the testing procedures and interpretation.
Testing Procedure and Preparation
Genetic testing involves blood, saliva, or cheek swab samples, although specialized laboratories may recommend different sample types.
A cheek swab or saliva sample is easily obtained from the comfort of home, while blood samples typically require a blood draw.
Normal Reference Ranges
Normal reference ranges for GDAP1 genetic testing are considered to be without mutations that can alter the activity of the GDAP1 proteins.
Clinical Implications of Positive GDAP1 Mutations
The clinical implications of a positive GDAP1 mutation test result will vary by individual. However, GDAP1 mutations in symptomatic patients may signal a need for further assessment and possibly treatment, especially in the setting of neuropathic symptoms.
Patients or practitioners with questions about the clinical implications of GDAP1 mutations should seek further assessment with a genetic counselor or expert.
What Does the Absence of Pathogenic GDAP1 Mutations Mean?
The absence of GDAP1 mutations does not rule out inherited neuropathies, as CMT is genetically heterogeneous. Many other genes are known to cause similar clinical presentations.
A negative GDAP1 result should prompt consideration of further genetic testing, particularly if there is a strong clinical suspicion of hereditary neuropathy.
Order Genetic Testing
Click here to compare genetic test panels and order genetic testing for health-related SNPs.





Entry - *606598 - GANGLIOSIDE-INDUCED DIFFERENTIATION-ASSOCIATED PROTEIN 1; GDAP1 - OMIM. (2015). Omim.org. https://omim.org/entry/606598
GDAP1 ganglioside induced differentiation associated protein 1 [Homo sapiens (human)] - Gene - NCBI. (2025). Nih.gov. https://www.ncbi.nlm.nih.gov/gene/54332
Gene Database. (2024). GDAP1 Gene - GeneCards | GDAP1 Protein | GDAP1 Antibody. Genecards.org. https://www.genecards.org/cgi-bin/carddisp.pl?gene=GDAP1
Miressi, F., Benslimane, N., Favreau, F., Rassat, M., Richard, L., Bourthoumieu, S., Laroche, C., Magy, L., Magdelaine, C., Sturtz, F., Lia, A.-S., & Faye, P.-A. (2021). GDAP1 Involvement in Mitochondrial Function and Oxidative Stress, Investigated in a Charcot-Marie-Tooth Model of hiPSCs-Derived Motor Neurons. Biomedicines, 9(8), 945. https://doi.org/10.3390/biomedicines9080945
Nagappa M, Sharma S, Taly AB. Charcot-Marie-Tooth Disease. [Updated 2024 Jun 22]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK562163/
Sivera, R., Frasquet, M., Lupo, V., García-Sobrino, T., Blanco-Arias, P., Pardo, J., Fernández-Torrón, R., de Munain, A. L., Márquez-Infante, C., Villarreal, L., Carbonell, P., Rojas-García, R., Segovia, S., Illa, I., Frongia, A. L., Nascimento, A., Ortez, C., García-Romero, M. del M., Pascual, S. I., & Pelayo-Negro, A. L. (2017). Distribution and genotype-phenotype correlation of GDAP1 mutations in Spain. Scientific Reports, 7(1). https://doi.org/10.1038/s41598-017-06894-6
Sutinen, A., Paffenholz, D., Tuyet, T., Salla Ruskamo, Torda, A. E., & Petri Kursula. (2023). Conserved intramolecular networks in GDAP1 are closely connected to CMT-linked mutations and protein stability. PLoS ONE, 18(4), e0284532–e0284532. https://doi.org/10.1371/journal.pone.0284532
Sutinen, A., Tuyet, T., Arne Raasakka, Gopinath Muruganandam, Loris, R., Ylikallio, E., Henna Tyynismaa, Luca Bartesaghi, Salla Ruskamo, & Petri Kursula. (2022). Structural insights into Charcot–Marie–Tooth disease‐linked mutations in human GDAP1. FEBS Open Bio, 12(7), 1306–1324. https://doi.org/10.1002/2211-5463.13422
Test for
GDAP1












